点击展开
Gene editing dog model
Gene editing dog model
Background Information
1Leber’s congenital amaurosis
Leber's congenital amaurosis (LCA) is the earliest and most severe hereditary retinopathy, in which the function of cone-rod cells in both eyes is completely lost at birth or within one year after birth, leading to congenital blindness in infants. LCA accounts for more than 5% of inherited retinopathy and is the leading cause of congenital blindness in children (10-20%). LCA is mostly autosomal recessive inheritance and clinically characterized by nystagmus, solid vision disorder, photophobia and fingerpress. Several pathogenic genes associated with LCA have been found, including RPE65, GUCY2D and CRX.
Bainbridge, of University College London, and Maguire, of the University of Pennsylvania, also found that subretinal injection of a recombinant adenoviral companion virus (AAV) vector containing RPE65 complementary DNA(cDNA) improved visual function in some patients with LCA. In 2001, cDNA fragments containing the human RPE65 encoding sequence and promoter region sequence were inserted from the retina into the Swedish Briard dog, a model animal with a natural RPE65 mutation, using recombinant AAV as a vector. With this gene therapy, the animal's visual function can be improved.
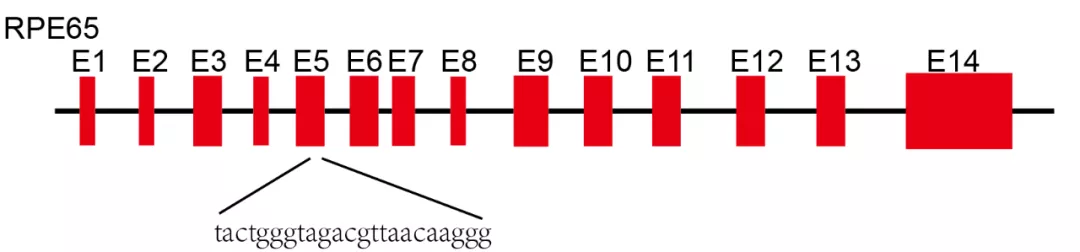
2Rpe65 gene
It's the first time on the Rpe65 gene knock out mice to study the gene function in the body, at the same time to establish a simulation model of LCA disease, but it is a pity that due to the species differences, human beings have rods and vertebral visual cells dysfunction, while mice lack of corresponding visual cells, thus phenotype in mice and human patients have clear distinction, that's why more suitable animal models are urgently needed . We used CRISPR/Cas9 to edit the Rpe65 gene exon 5, and finally obtained a +7bp homozygous model dog.
Results of F0 generation of model dogs
1Electrophysiological abnormality

Mutant dogs showed no response to photostimulation signals at 2 and 4 months of age (data unpublished)
2Model application
1. Use model dogs to carry out relevant research on the pathogenesis of congenital amaurosis through DNA, RNA molecular level, electrophysiological level and other aspects;
2. Use model dogs to conduct drug effectiveness experiments and evaluations;
3. Use model dogs to carry out relevant research and develop new drugs;
4. Use model dogs to conduct non-drug therapy effectiveness experiment and evaluation, and innovate and develop disease treatment methods:
1. Evaluate the therapeutic effect of stem cells on congenital amaurosis;
2. Evaluate the therapeutic effect of gene therapy on congenital amaurosis, etc.